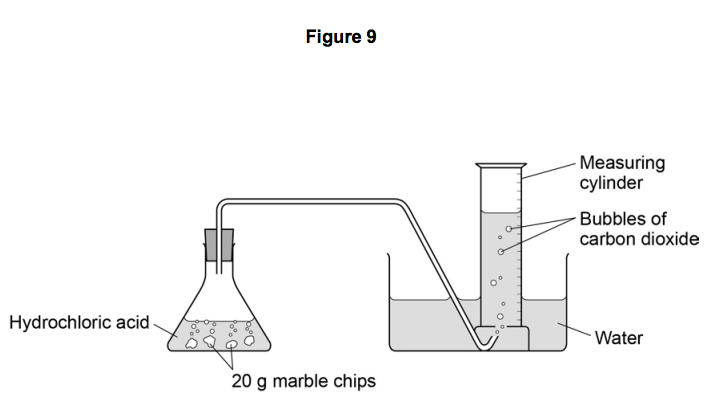
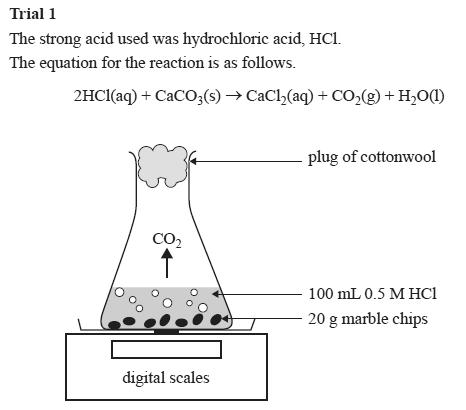
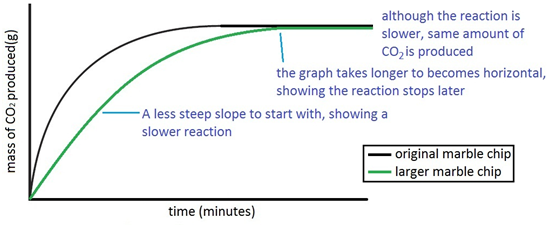
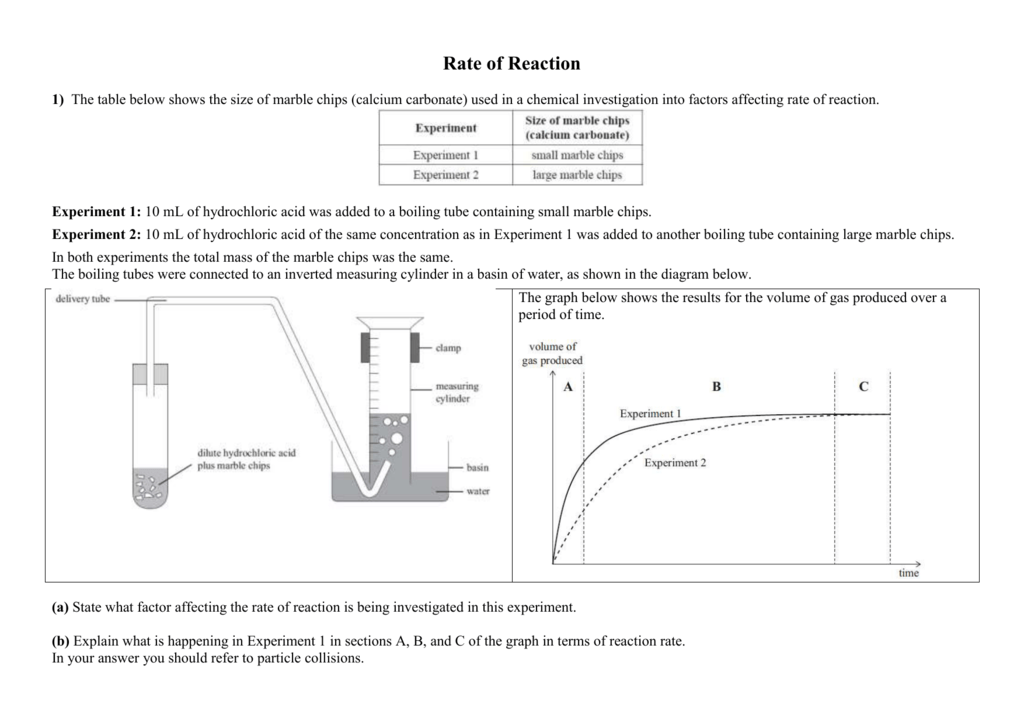
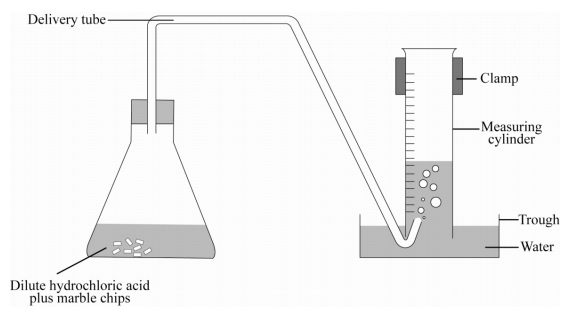
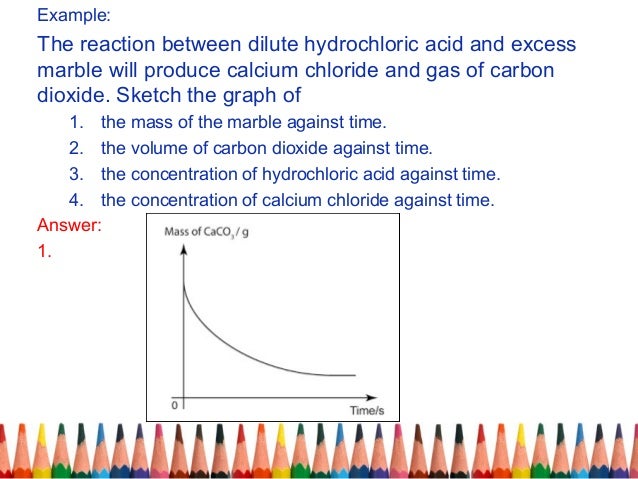
Cacl2 aq h2o l co2 g in this experiment i am going to see if temperature affects the reaction rate between marble chips and hydrochloric acid by timing the release of carbon dioxide in the reaction.
Marble hcl reaction.
Calcium carbonate is dissolved by hydrochloric acid thereby forming gaseous carbon dioxide.
Hydrochloric acid or hcl is a strong acid that donates h to water.
With the equation caco3 2hcl cacl2 h2o co2 hypotheses a reaction occurs when particles collide.
Marble reaction with hydrochloric acid drop a small amount of dilute hydrochloric acid on an area of your sample that has been scratched by a nail.
This is because there will be more hydrochloric acid particles to collide with the marble chip particles.
Marble chips are mostly made up of calcium carbonate which is a alkaline compound.
The higher the concentration of hydrochloric acid in the beaker the faster the reaction will take place.
In fact if you take baking soda which contains hco3 and add vinegar which donates h to the solution this is precisely what happens.
The increased h increases the rate of the reverse reactions forming carbon dioxide and water.
Calcium carbonate and hcl.
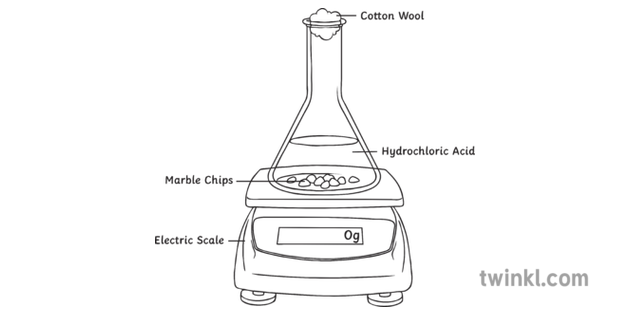
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
Caco3 s 2hcl aq 61614.
Marble is crystalized caco3.
Therefore resulting in a quicker reaction.
Detailed description pdf video mpg4.
This process is based on random particle movement.
The reaction takes place spontaneously.
There are many variables that affect.
Being alkaline it reacts with hydrochloric acid to produce calcium chloride water and carbon dioxide.
The overall reaction is 2hcl caco3 cacl2 co2 h2o.
Marble chips and hydrochloric acid planning aim to find if changing the concentration of an acid will increase or decrease the rate of the reaction when marble is dissolved in hydrochloric acid.
Click each image to see positive and negative results of the acid test.
Pieces of marble are thrown into hydro chloric acid.